Ejuna SyncSleep (DTX)
Realising a new product to cure chronic insomnia
Ejuna SyncSleep enables patients to personalise the timing of melatonin, maximising its sleep-inducing effects, and engage in cognitive behavioural therapy for insomnia (CBT-I) to cure chronic insomnia for the long term.
Role
As Director of Product Design, I led Ejuna SyncSleep’s design and development for Closed Loop Medicine. This spanned early scoping, ideation, design, medical-device certification, and submission for regulatory approval. The result was a new, viable treatment for insomnia.
Deliverables
Product definition & vision
UI & UX design
Packaging design
Service design
Usability Engineering File
Design and development capability
Outcomes
UKCA and CE Medical Device Certification
13M in funding secured
MHRA combination product submitted
Partnership secured with Teva
Digitising Therapy
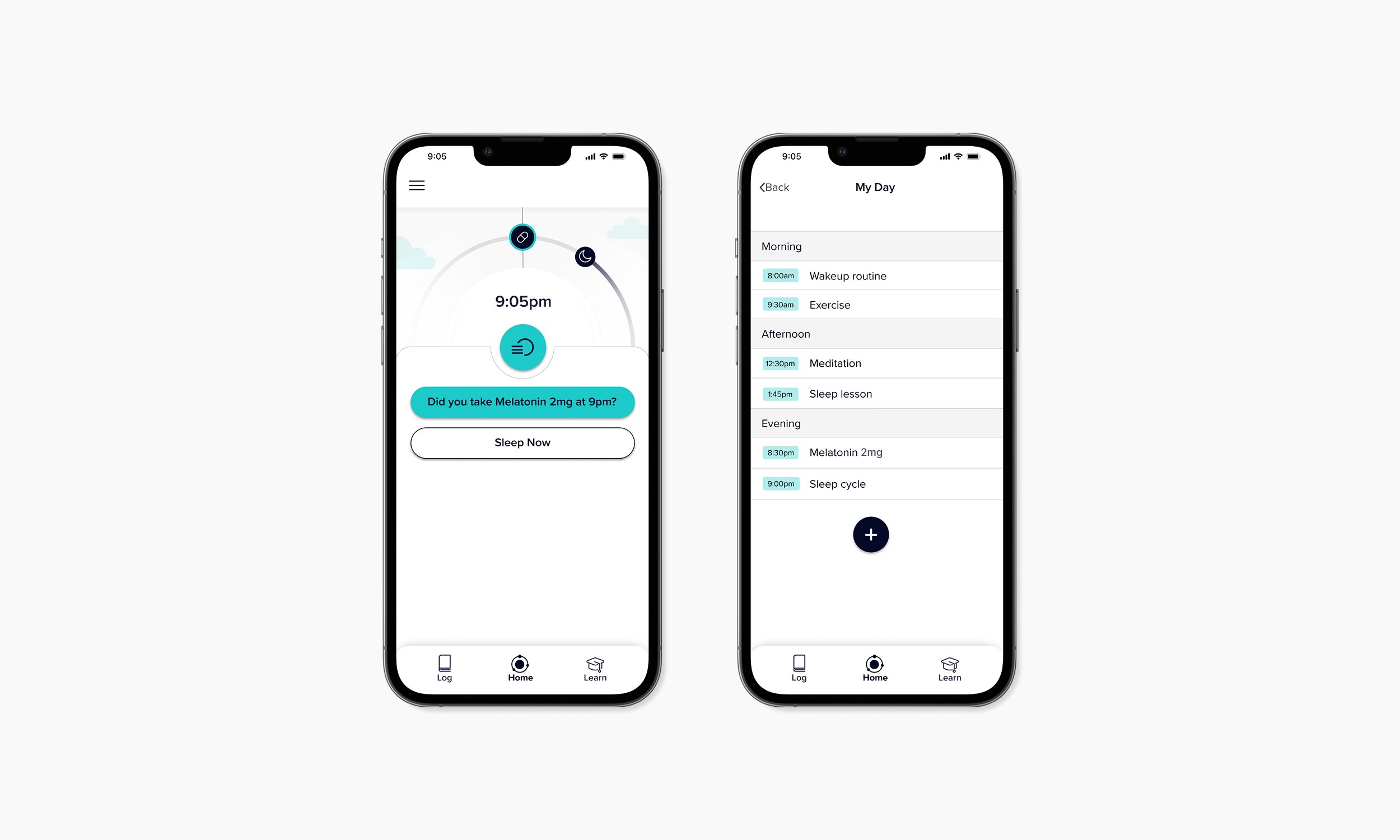
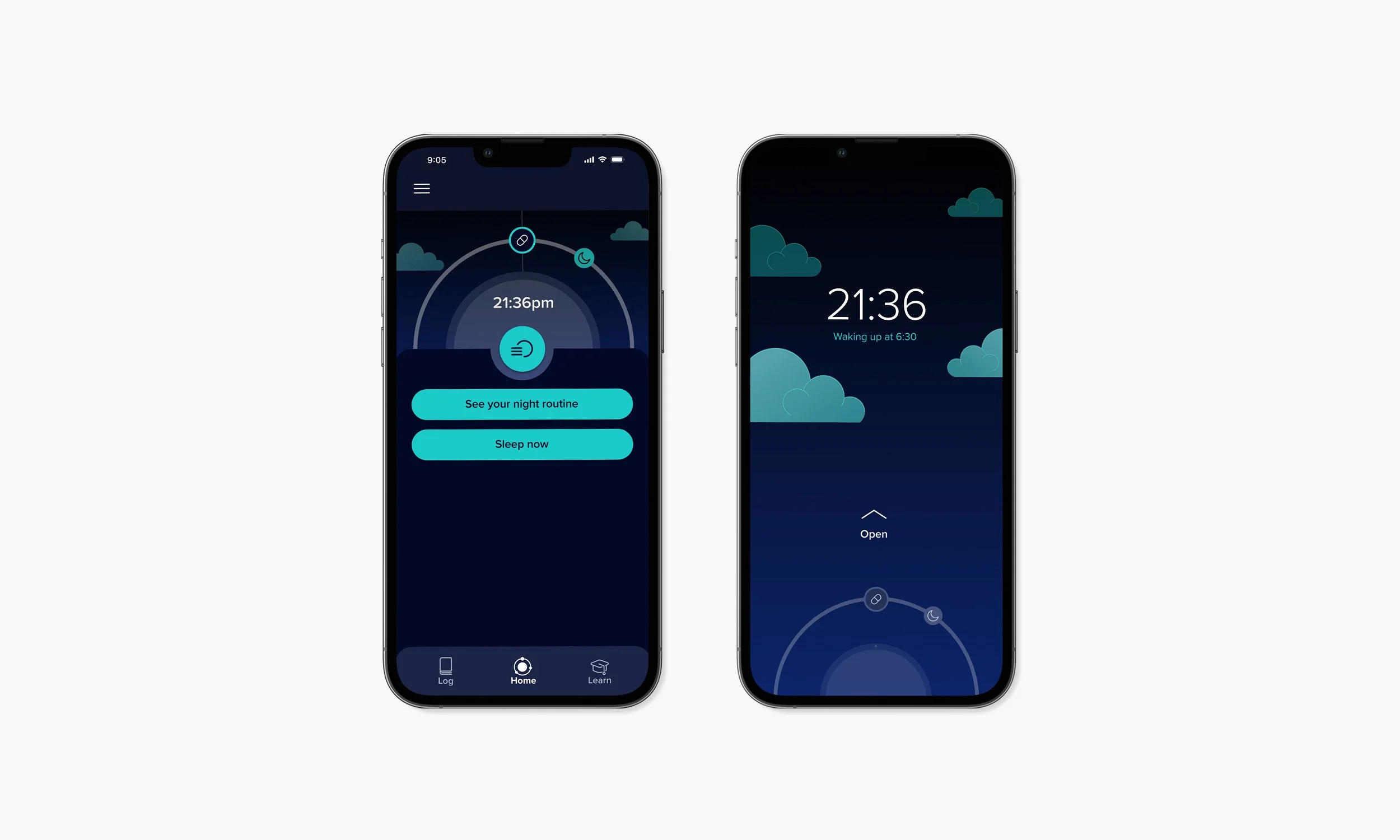
Patients learn how to to implement effective therapy to cure insomnia. The experience designed offers patients a comprehensive way to time their medication taking, engage with CBTi learning content, implement new behaviours, therapeutic interventions and gain confidence by tracking progress and see that the treatment is working.
Working closely with patients and clinicians, I attended therapy sessions using cognitive behavioural therapy for insomnia, collaborated with healthcare professionals to develop a learning programme, ran behaviour change workshops to define the interaction approach, designed the interface with the product team, and validated the solution by testing with patients.
Integrating product onboarding
Digital therapy is accessed directly on receipt of the medication package with no need for any other sign up procedure. This reduced friction in onboarding, ensured contextual relevance at the point of use, and increased user engagement by delivering access precisely when and where it was needed.
Assembling a cross-functional team that included regulatory experts, a packaging design agency, and a UI engineer, I led the development of this solution. We researched regulatory constraints, explored methods for restricting app access, consulted pharmaceutical manufacturers to assess feasibility, developed packaging and app prototypes, then tested and iterated onboarding concepts with patients.
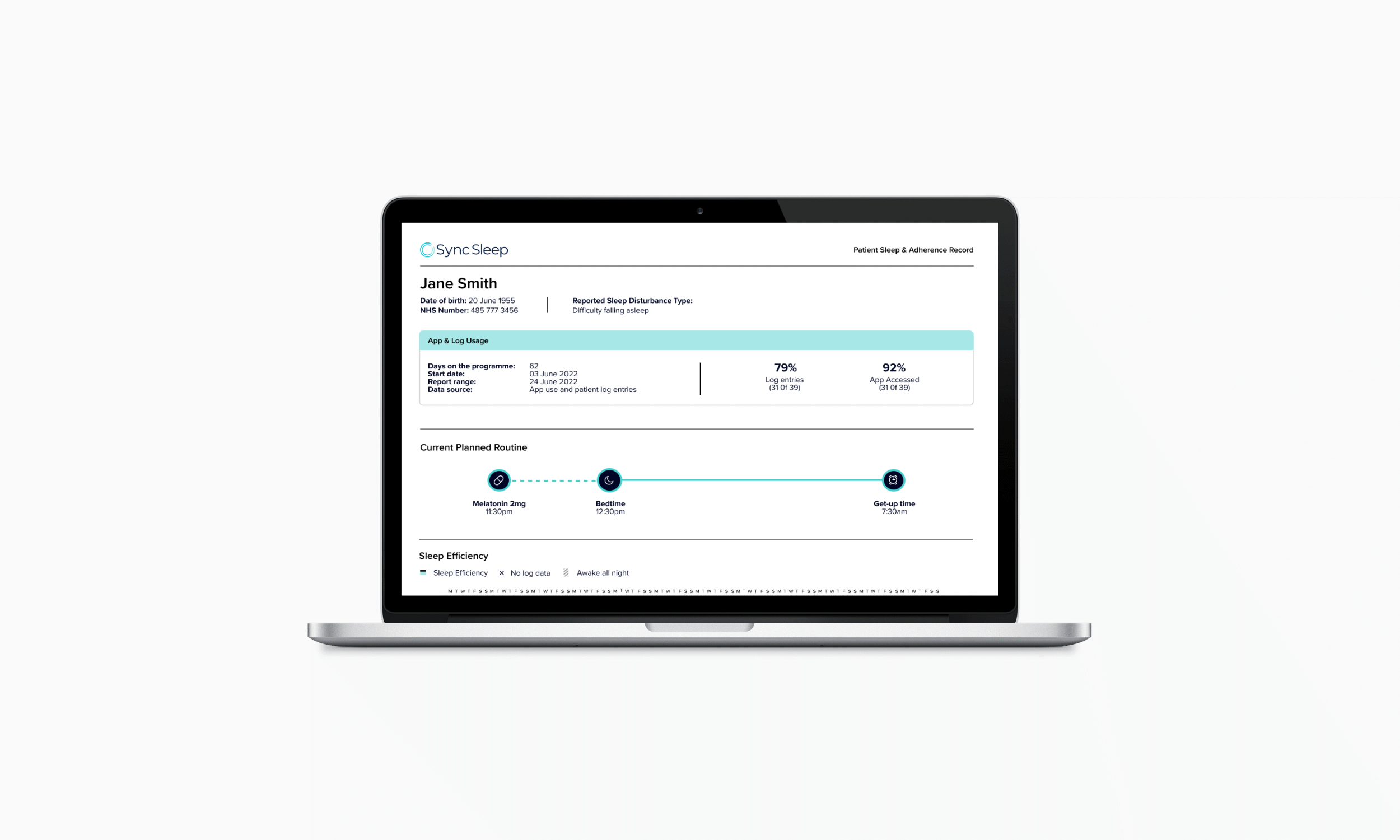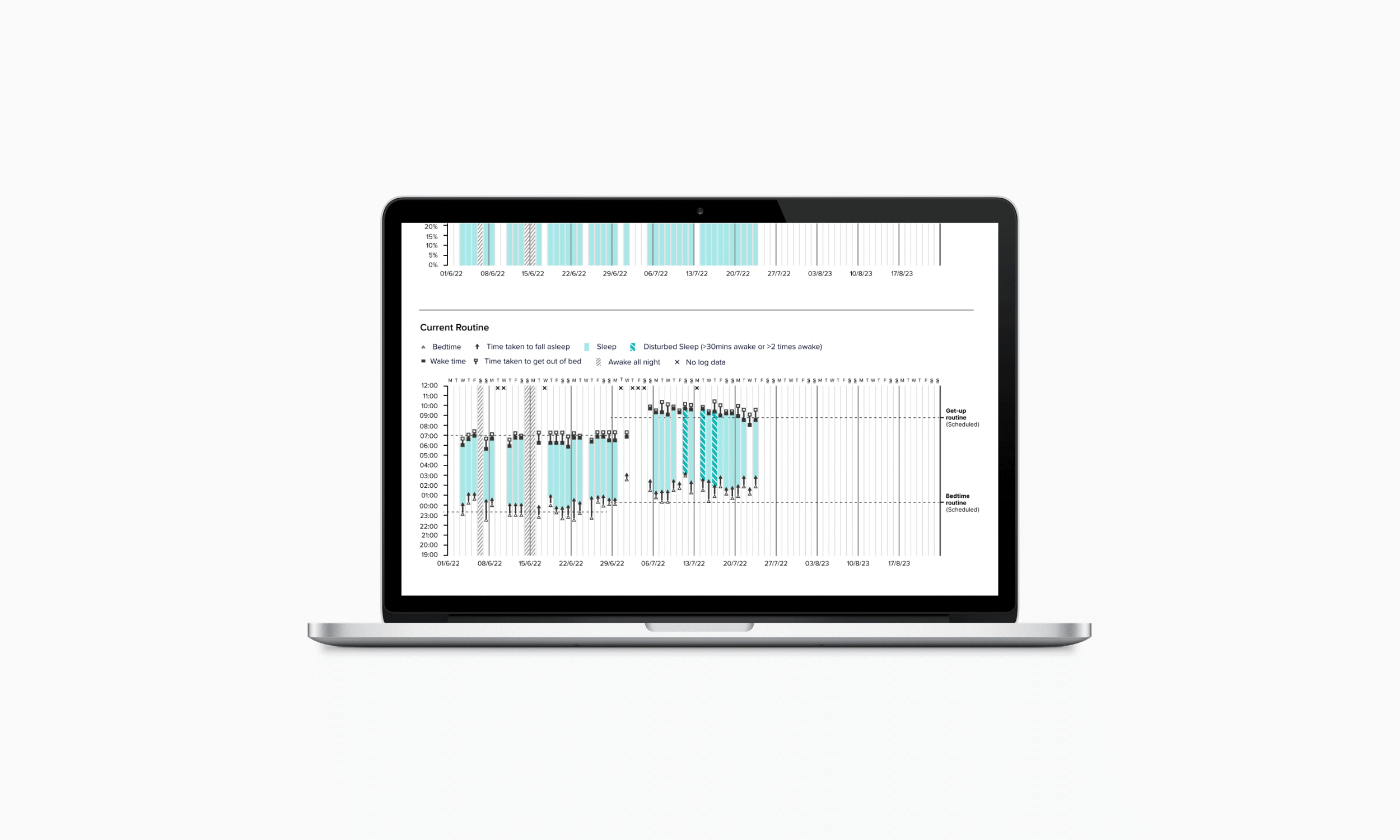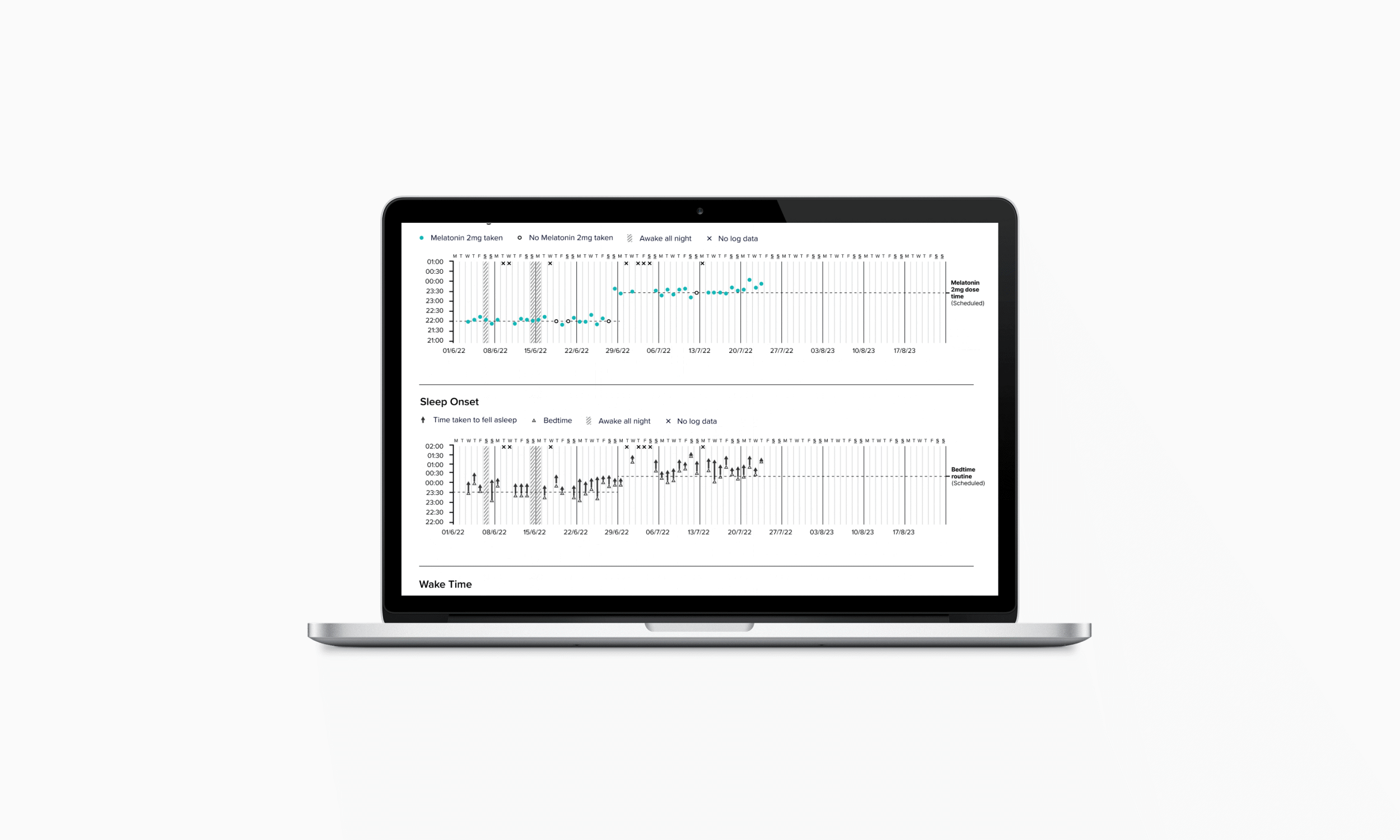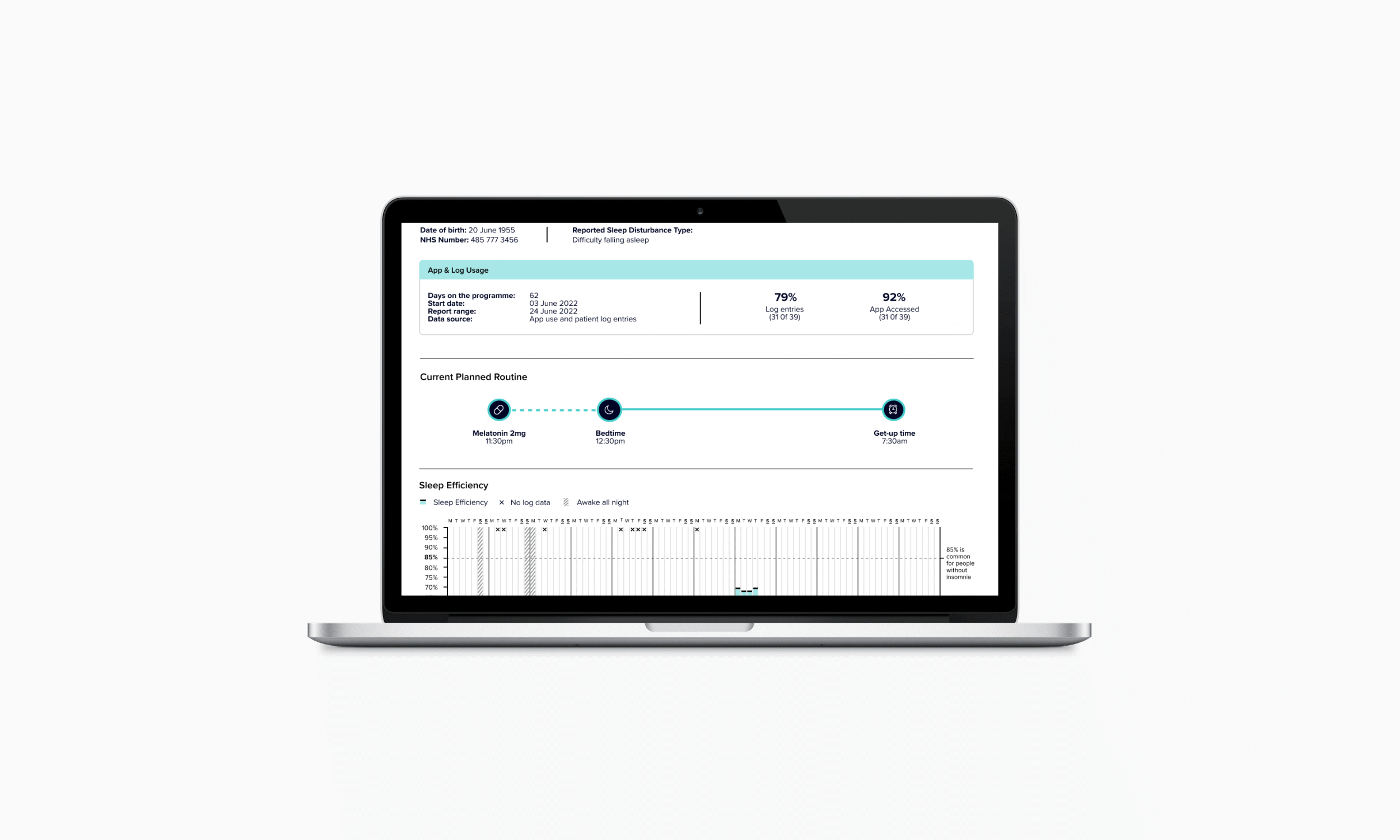
Personalised Treatment Decisions
Clinicians gain a clear view of patient progress through a simple interface that highlights behavioural patterns and medication adherence. The experience design supports treatment adjustments, enabling tailored dose timing for different types of insomnia, including delayed sleep onset, night-time waking, and early waking.
In creating the clinical decision-making report, I collaborated with CLM’s chief clinical officer and two external sleep therapists to define the assessment logic, translated this into data visualisations, worked with designers and developers to create feasible solutions, tested and refined the interface with clinicians, and developed clinical instructions to support training for rollout.
Engagement using Effective Behaviour Change Strategy
Beneath the patient experience lies a carefully crafted engagement strategy designed to support vulnerable individuals who might easily drop off therapy. By introducing creative methods to tackle their condition, patients are encouraged to learn, implement, and track their progress as they actualise their therapy journey.
Shaping the product strategy involved analysing the cognitive behavioural therapy programme with sleep therapists, conducting workshops using the taxonomy of behaviour change, and mapping a prioritised set of techniques into an intuitive system architecture and interaction design for patients.
Medical Device Certification
To attain market access as a single prescription combination product, it was essential to certify the app as a medical device. This was achieved by partitioning any safety-related features and getting them approved separately, allowing for rapid and continuous improvement of the non-safety-related content in the CBT-I program.
For the UKCA and CE medical device approval, my role centred on developing the usability engineering file, writing the use specification, conducting a UFMEA risk analysis, designing controls, and coordinating evaluations with a third-party moderator.
Project Data
User need
The NHS estimates that about 30% of adults experience insomnia or significant sleep disruption, with around 6–7% developing chronic insomnia, which can precipitate or exacerbate anxiety, depression, and reduced quality of life.
Context
Patients and providers are underserved by limited access to effective treatments such as CBT-I, and symptom-relieving medicines like melatonin are often improperly timed or administered.
Value proposition & business case
Combining digital CBT-I with melatonin enables an effective treatment to be delivered through existing pharmaceutical supply chains, increasing access at a reduced cost compared with stand-alone services.
Technologies
CBT-I; melatonin; behavioural science; dose-timing algorithms; patient-facing smartphone application; clinician-facing web application; AWS back-end services.